Description
Toluene is also used in the production of polymers used to make nylon, plastic soda bottles, and polyurethanes and for pharmaceuticals, dyes, cosmetic nail products, and the synthesis of organic chemicals.
Of the three compounds has a central benzene ring with two methyl groups attached at substituents.
Toluene has been reported as the most commonly abused hydrocarbon solvent, primarily through ‘glue sniffing’. The common possibilities of exposure to high levels of toluene include indoor air from the use of household products such as paints, paint thinners, adhesives, synthetic fragrances, and many other sources.
Toluene (molecular formula: C7H8) is a homologue of benzene, also known as “methyl benzene” and “phenyl methane”. It is a colorless, volatile liquid with a special aroma. Toluene is a member of aromatic hydrocarbons. In the air, toluene can only incompletely burn and the flame is yellow. Many of its properties are similar to those of benzene and have aromatic odors similar to those of benzene. In practice, they are often used as organic solvents instead of the rather toxic benzene.
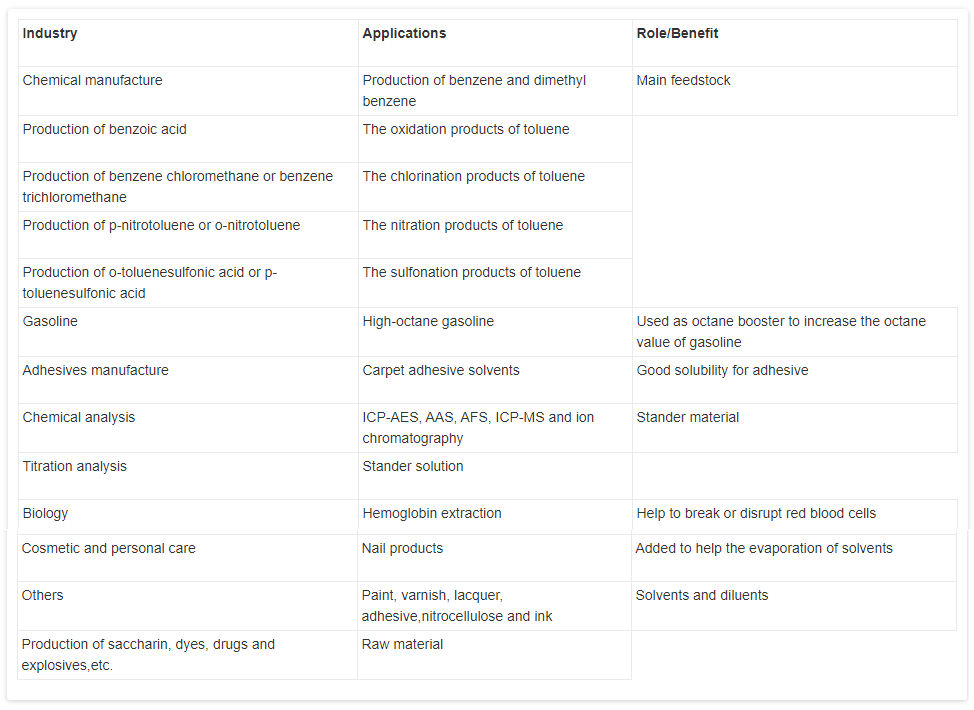
Toluene is prone to chlorination and produces benzene-chloromethane or benzene-trichloroethane, both of which are good industrial solvents; it can extract bromine from the bromine water, but cannot react with bromine water; it is also easy to nitrify and produce nitrotoluene or o-nitrotoluene, both of which are the raw materials of dyes; one part of toluene and three parts of nitric acid are nitrated to give trinitrotoluene (common name TNT); it is also easily sulfonated to generate o-toluene sulfonic acid or p-toluene sulfonate which are the raw materials for making dyes or making saccharin. Toluene vapor mixes with air to form explosive substances, so it can make TNT explosives.
Toluene Uses
- Toluene is a component of gasoline, paints, inks, lacquers, paint thinners, adhesives, fingernail polish, cleaning agents, and rubber. BTX (a mixture of benzene, toluene, and xylene) is added to gasoline to improve octane ratings. Toluene is used to produce benzene, trinitrotoluene (TNT), nylon, plastics, and polyurethanes. It is also used in production of drugs of abuse. Toluene is a favorite of solvent abusers, who intentionally inhale high concentrations to achieve a euphoric effect. It is used in the production of pharmaceuticals, dyes, and cosmetic nail products. It is used against roundworms and hookworms.
- Toluene is derived from coal tar as well as petroleum. It occurs in gasoline and many petroleum solvents. Toluene is used to produce trinitrotoluene (TNT), toluene diisocyanate,and benzene; as an ingredient for dyes, drugs, and detergents; and as an industrial solvent for rubbers, paints, coatings, and oils.
- Toluene has numerous applications in the chemical and petroleum industry, with approximately 6 million tons used annually in the United States and 16 million tons used globally. The major use of toluene is as an octane booster in gasoline. Toluene has an octane rating of 114. Toluene is one of the four principal aromatic compounds, along with benzene, xylene, and ethylbenzene, that are produced during refining to enhance gasoline’s performance. Collectively, these four compounds are abbreviated as BTEX. BTEX is a major component of gasoline, forming about 18% by weight of a typical blend. Although the proportion of the aromatics is varied to produce different blends to meet geographic and seasonal requirements, toluene is one of the major components. A typical gasoline contains approximately 5% toluene by weight.
Toluene is a primary feedstock used to produce various organic compounds. It is used to produce diisocyanatos. Isocyanates contain the functional group ?N = C = O, and diisocyanatescontain two of these. The two main diisocyanatos are toluene 2,4-diisocyanate and toluene 2,6-diisocyanate. The production of diisocyanatos in North America is close to a billionpounds annually. More than 90% of toluene diisocyanato production is used for makingpolyurethanes foams. The latter are used as flexible fill in furniture, bedding, and cushions. IN rigid form it is used for insulation, hard shell coatings, building materials, auto parts, and roller skate wheels. - In manufacture of benzoic acid, benzaldehyde, explosives, dyes, and many other organic Compounds; as a solvent for paints, lacquers, gums, resins; thinner for inks, perfumes, dyes; in the extraction of various principles from plants; as gasoline additive.
- Formula: C7H8
- Density: 867 kg/m³
- Boiling point: 110.6 °C
- Molar mass: 92.14 g/mol
- Melting point: -95 °C
- Classification: Aromatic compounds
- Vapor density: 3.2 (vs air)
- Vapor pressure: 22 mm hg ( 20 °c)
- Refractive index n/d: 1.496(lit.)
- Flash point: 40 °f
- Storage temp: 0-6°c
- Pka: 40(at 25℃)
- Form: liquid
- Specific gravity: 0.865~0.870(20/20℃)(ph.eur.)
- Color : colorless
- Odor: Aromatic, Benzene-like odor detectable at 0.16 to 37 ppm (mean = 1.6 ppm)
- Relative Polarity: 0.099
|
Signal word |
Danger |
|
Hazard statements |
H225-H304-H315-H336-H361-H373-H302-H333 |
|
Precautionary statements |
P210-P260-P301+P310a-P303+P361+P353-P405-P501a |
|
Hazard Codes |
|
|
Risk Statements |
11-38-48/20-63-65-67-39/23/24/25-23/24/25 |
|
Safety Statements |
36/37-46-62-45-16-7 |
|
RIDADR |
UN 1294 3/PG 2 |
|
WGK Germany |
2 |
|
RTECS |
XS5250000 |
|
F |
03-Oct |
|
Auto ignition Temperature |
480 °C |
|
TSCA |
Yes |
|
Hazard Class |
3 |
|
Packing Group |
II |
|
HS Code |
29023000 |
|
Toxicity |
LD50 orally in rats: 7.53 g/kg (Smyth) |
Hazards & Heath Effects
Metabolism in body
80% of Toluene absorbed in the body is oxidized to benzyl alcohol in the presence of NADP (transconjugase II), oxidized to benzaldehyde in the presence of NAD (transconjugase I), and then oxidized to benzoic acid. Then, it is combined with glycine to form hippuric acid in the presence of adenosine triphosphate. Therefore, 16%-20% of toluene absorbed into the body is exhaled by the respiratory tract, and 80% are excreted in the form of hippuric acid through the kidneys.
Human Health Effects
People are most likely to be exposed to toluene by smoking or using consumer products containing toluene (paints, varnish, nail polish, paint cleaners, stain removers, etc.) especially if there is not good ventilation.
Eye and upper airway irritation occurred after a 6.5 hr exposure to an air level of 100 ppm (377 mg/cu m) toluene, and lacrimation was seen at 500 mg/cu m. Volunteers exposed to 100 ppm (377 mg/cu m) toluene for 6 hr/day for four days suffered from subjective complaints of headache, dizziness and a sensation of intoxication. In subjects exposed to 750 mg/cu m for 8 hr, fatigue, muscular weakness, confusion, impaired coordination, enlarged pupils and accommodation disturbances were experienced; at about 3000 mg/cu m, severe fatigue, pronounced nausea, mental confusion, considerable in coordination with staggering gait and strongly affected pupillary light reflexes were observed. After exposure at the high level, muscular fatigue, nervousness and insomnia lasted for several days. Heavy accidental exposure leads to coma.
There is inadequate evidence for the carcinogenicity of toluene in humans. There is evidence suggesting lack of carcinogenicity of toluene in experimental animals. Therefore, Toluene is not classifiable as to its carcinogenicity to humans.
Chemical Properties
Also known as methyl benzene or phenyl methane, C6H5CH3 is a flammable, toxic, colorless liquid. Insoluble in water and soluble in alcohol and ether, it is used in explosives, high-octane gasoline, and organic synthesis.
Chemical Properties
Toluene is a clear, colorless, flammable liquid with a sweet/pungent odor. It is extensively used as a solvent in different industries, i.e., rubber chemical manufacturing, drugs and pharmaceuticals, thinner for inks, paints dyes, and perfume manufacturing. It is a natural constituent of crude oil and is produced from petroleum refi Ning and coke-oven operations. Toluene occurs naturally as a component of crude oil and occurs in petroleum refi Ning and coke oven operations. Occupational workers associated with several kinds of activities, such as manufacturing of dyes, printing inks, painting automobile mechanics, gasoline manufacturers, shippers, and retailers, adhesives and coatings manufacturers and applicators, audio-equipment product workers, chemical industry workers, coke-oven workers, fabric manufacturers (fabric coating), sites of hazardous wastes, linoleum manufacturers, in pharmaceutical manufacturing, printing works, shoe manufacturing industry, become exposed to toluene.
Chemical Properties
Toluene is a clear, colorless, noncorrosive liquid with a sweet, pungent, benzene-like odor. The Odor Threshold in air is variously given as 0.17 ppm, 2.9 ppm (NJ) and 8 ppm (EPA). The Odor Threshold in water is 0.04-1.0 mg/L
Physical properties
Colorless, clear, flammable liquid with a pleasant, sweet or paint-like odor similar to benzene. At 40 °C, the lowest concentration at which an odor was detected were 960 μg/L. Similarly at 25 °C, the lowest concentration at which a taste was detected was 960 μg/L (Young et al., 1996). Experimentally determined detection and recognition odor threshold concentrations were 600 μg/m3 (160 ppbv) and 7.0 mg/m3 (1.9 ppmv), respectively (Hellman and Small, 1974). Leonardo’s et al. (1969) reported higher odor threshold concentrations for toluene derived from coke (4.68 ppmv) and petroleum (2.14 ppmv). The average least detectable odor threshold concentrations in water at 60 °C and in air at 40 °C were 24 and 140 μg/L, respectively (Alexander et al., 1982). An odor threshold concentration of 330 ppbv was determined by a triangular odor bag method (Nagata and Takeuchi, 1990). Cometto-Mu?iz and Cain (1994) reported an average nasal pungency threshold concentration of 29,574 ppmv.
History
Toluene is a clear, flammable, aromatic hydrocarbon liquid with a smell similar to benzene. It is also called methylbenzene, indicating that a methyl group has been added to one of benzene’s carbon atoms. Toluene was first isolated by Pierre-Joseph Pelletier (1788–1842) and Philippe Walter (1810–1847) in 1837. The name toluene comes from the South American tree Toluifera balsamum. Henri-Etienne Sainte-Claire Deville (1818–1881) isolated toluene from the tree’s gum, Tolu balsam, in 1841.
The main source of toluene is from the catalytic reforming of naphtha’s during petroleum processing. During this process cycloalkanes are dehydrated, forming aromatics such as toluene and xylene along with hydrogen. Toluene can also be obtained from the pyrolysis of gasoline. It is a by-product when styrene is produced and can also be produced from coal tar, which was its main source in the first half of the 20th century.








Reviews
There are no reviews yet.